Abstract
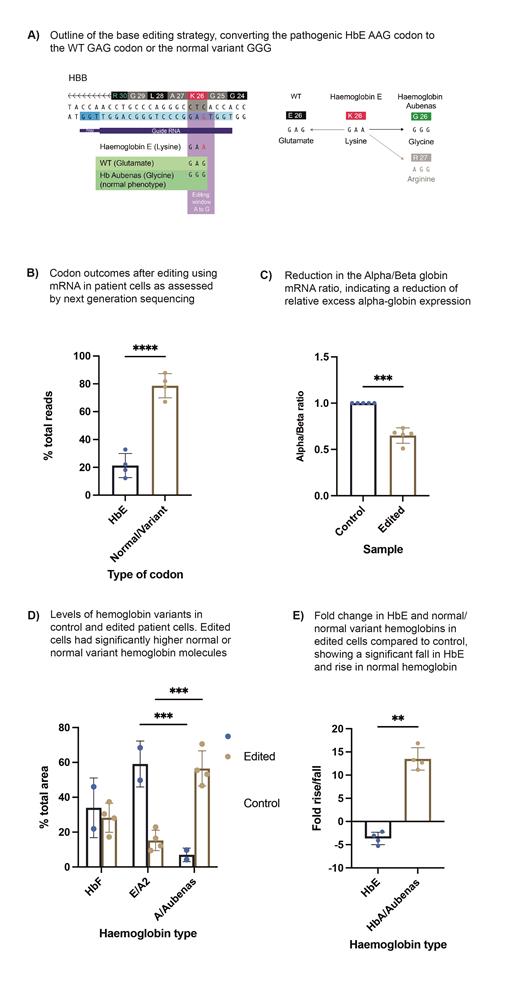
HbE/β-thalassemia is the commonest form of severe β-thalassemia, and comprises approximately 50% of all cases worldwide. HbE/β-thalassemia is caused by the HbE codon 26 G>A mutation on one allele and any severe β 0-thalassemia mutation on the other. These mutations lead to a reduction in β-globin production, resulting in a relative excess in α-globin chains that go on to cause ineffective erythropoiesis. Importantly, individuals with a mutation on one, but not two, alleles have β-thalassemia trait, a carrier state with a normal phenotype. Recent gene therapy and gene editing approaches have been developed to treat β-thalassemia but do not directly repair the causative mutation in-situ. Gene replacement approaches rely on lentiviral vector-based sequence insertion or homology directed repair (HDR). HbF induction strategies also rely on non-homologous end joining (NHEJ) targeting of enhancers in-trans. These approaches, whilst variably successful, are associated with potential safety concerns. Adenine base editors (ABEs) potentially circumvent these problems by directly repairing pathogenic variants in-situ through deamination. ABEs catalyse A-T to G-C conversions through targeting with a Cas9-nickase and single-guide RNA (sgRNA). Conversion of the HbE codon to normal through base editing is an attractive strategy to recapitulate the phenotypically normal β-thalassemia trait state without potentially harmful double-strand breaks or random vector insertions (Figure 1A). ABEs are able to convert the HbE codon (AAG, lys) to wild-type (GAG, glu), but also to GGG (gly) or AGG (arg). GGG at codon 26 is found in a naturally occurring hemoglobin, Hb Aubenas. Heterozygotes have normal red cell indices and are phenotypically normal.
We electroporated the latest generation of ABE8 editors (ABE8e, ABE8.13 and ABE8 V106W) as mRNA into WT CD34+ hematopoietic stem and progenitor cells (HSPCs) with sgRNAs targeting the middle A of the WT GAG codon. These had similar editing efficiencies although ABE8 V106W had marginally higher on-target efficiency. V106W has been evolved to have a favourable off-target profile. V106W mRNA/sgRNA was electroporated into 3 different severe HbE/β-thalassemia donor HSPCs. The HbE codon was converted to WT with a mean 28.7% efficiency, to Hb Aubenas 48.6% and to an undescribed AGG codon 2.1%. The mean conversion from HbE to a normal or normal variant was 78.7±8.7% (Figure 1B). The indel rate from inadvertent on-target Cas9 cleavage was below 0.5%. Edited cells did not show any perturbations in erythroid differentiation as assessed by Immunophenotyping and cellular morphology. In differentiated erythroid cells, RT-qPCR showed a mean fall in the α/β mRNA ratio to 0.65±0.08 (unedited patient cells normalised to 1, n=5, Figure 1C), indicating a reduction in the relative excess α-globin gene expression. Protein analysis by CE-HPLC showed a 3.6-fold reduction in HbE levels (SD±1.3) and a 13.5-fold increase in HbA/Hb Aubenas (SD±2.4, Figure 1C and D).
To prove that base editing using mRNA was possible in long-term HSCs, CD34+ cells from 4 WT cord blood donors were edited using ABEmax. Mice were culled after 16 weeks, and human cells were collected and transplanted into 7 secondary mice, which were also culled after 16 weeks. Each secondary mouse showed the presence of hCD45+ cells, indicating engraftment of LT-HSCs. All secondary replicates showed editing, with a mean editing efficiency of 34.5% (initial editing 46.3%). In both rounds of mice, there was robust lymphoid and myeloid engraftment and expected levels of erythroid engraftment for the NSG model in bone marrow and spleen. Potential off-target effects were assessed in-vitro by CIRCLE-seq in triplicate. These sites were assessed by targeted oligonucleotide capture of DNA from mRNA edited patient cells to detect in-vivo editing.
Together these data provide robust evidence for base editing as an effective and safe therapeutic strategy for HbE/β-thalassemia.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal